

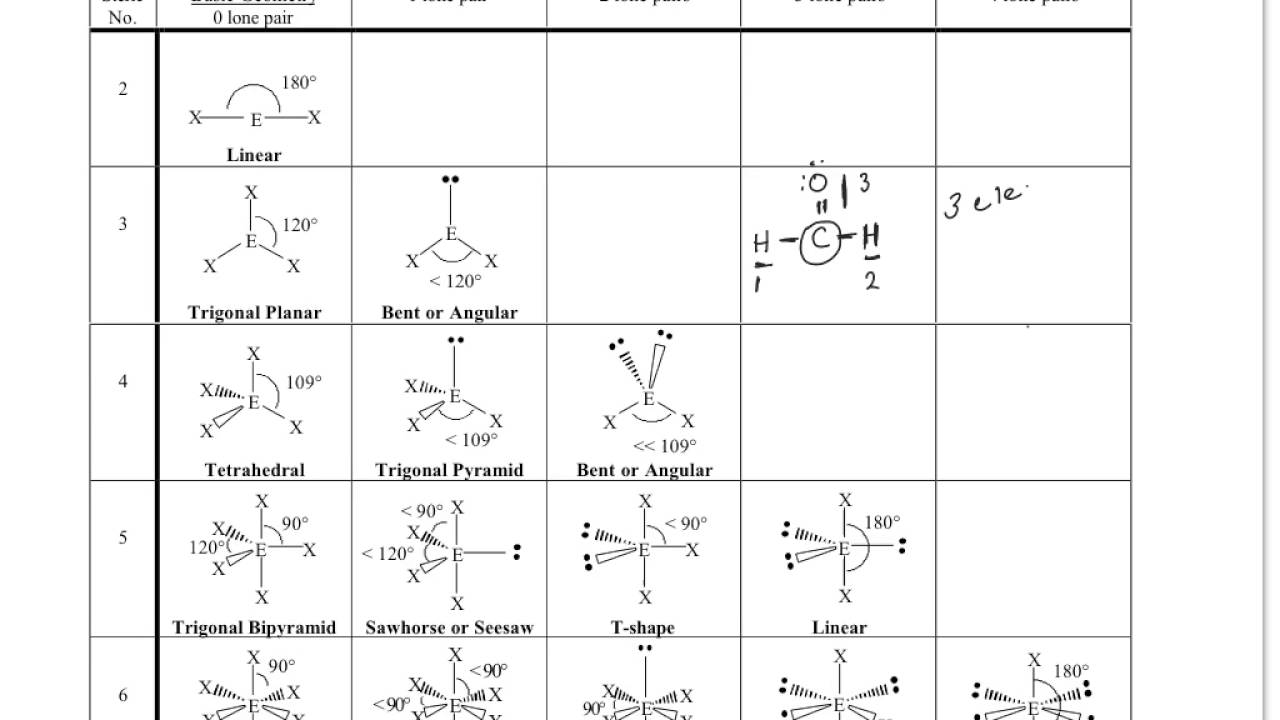
Firstly, we must know how many total attachments there are. To determine the molecular geometry of a structure we need to know two things. Configurationĭetermining molecular geometry and bond angles Below is a table demonstrating the relationship between the number of bonding partners and these configurations. There are three main types of configurations: linear, trigonal, and tetrahedral. This theory revolves around the idea that electrons repel each other and therefore will bond accordingly. Chemists are able to predict the arrangement of atoms and chemical bonds using the valence-shell electron-pair repulsion theory or VSEPR. Molecular geometry describes the three-dimensional structure of a molecule. Bond angles: The angle between adjacent bonds of an atom.Hybridization: Orbitals are combined in order to spread out electrons.Molecular Geometry: Describes the arrangement of atoms around the central atom with acknowledgment to only bonding electrons.Electron Geometry: Describes the arrangement of bonds and lone pairs around a central atom.
MOLECULAR GEOMETRY FREE
If you enjoy this tutorial, feel free to check out our other tutorials on bonding listed below.
You will learn about the more common molecular geometries: tetrahedral, linear, bent, trigonal pyramidal, and trigonal planar – along with their bond angles.
MOLECULAR GEOMETRY HOW TO
Hence, the Carbon atom has sp hybridization in the C 2 H 2 molecule.In this tutorial, you will learn how to identify the molecular geometry and bond angles of a molecule. These two orbitals form two pi bonds that result in the formation of triple bonds between carbon atoms. There are two-half filled 2p orbitals for each Carbon atom. C-H bonds are created when the second sp orbital overalls with the 1s orbital of the Hydrogen atom.
This is the same for the other set of CH molecules too.Ĭarbon atoms form sigma bonds with each other when one sp orbital overlaps with the sp orbital of the other CH molecule. There is the formation of one hybridized s and p orbital. The 1s orbital of the Hydrogen atom overlaps with the Carbon atom’s 2p orbital atom, making it an sp hybridization. This hybridized molecule accommodates the shared electron of the Hydrogen. Carbon atom’s electron configuration in its ground state is 1s2 2s2 2p2, but when it is in its excited state, the electron from the 2s orbital moves to the 2pz orbital. To make it easier for you to understand, consider this molecule made up of two CH atoms. To attain a stable structure, the Carbon atoms will share their remaining three valence electrons by forming a triple bond. The octets of both the Hydrogen atoms are now complete, but Carbon atoms still don’t have a complete octet. Here, both the Hydrogen atoms will share one valence electron of the Carbon atom and form a bond. If you look at the Hydrogen atoms, it only needs one valence electron to attain a stable structure. Here both the Carbon atoms take the central position, and the Hydrogen atoms are arranged around it. The electrons that participate in forming bonds are called bonding pairs of electrons while the ones that do not take part in any bond formation are called lone pairs or non-bonding pairs of electrons.įor C 2 H 2 Lewis structure, we will first place both the Carbon atoms in the centre as it is less electronegative than the Hydrogen atoms. Lewis Structure of any molecule helps to know the arrangement of all atoms, their valence electrons, and the bond formation in the molecule.


 0 kommentar(er)
0 kommentar(er)
